|
|
 Japan Water Guard |
 |
| |
| ●To conserve 21st century environment and water front●
Save our Earth!! |
| | HOME | About Us | Activities | Water Study Room | Projects | Site Map | Link |
  |
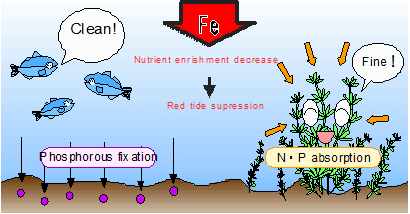
Seaweed bed ProjectTo grow seaweeds, nutrient sources for fertilizers such as nitrogen, phosphorous, and silicon are needed. In polluted sea water, since there are nitrogen and phosphorous, it tends to become nutrient enrichment. However, in the water, nitrogen are NO3- and phosphorous are PO4-, so that these substances don't become nutrient for seaweeds. In order them to be nutrient sources, it should return to Nitrogen and Phosphorous by reduction. Reduction is opposite of oxidation, so that it is chemical reaction to remove oxygen. Ferrous ion Fe+ is the metal ion which works for reduction. Ferrous ion is the main substance which occupies approximately 35% of the earth and 5% of crustal materials. Ferrous ion is typically easy to change its oxidation number from 2 to 3 on the surface of the earth and in organism body depending on oxidation-reduction environment change. Thus, in production and decomposition of organic mattes, it works important function. Although especially tervalent iron tends not to dissolve in the water so that there is no problem in the grand (it is problem for organisms on the ground when iron dissolves in organic matters or becomes acid), lack of iron is serious issue in the ocean. The form which seaweeds easily to absorb Iron is "fulvic acid" which is produced by leaf molds. Fulvic acid makes iron under the ground dissolve in the ocean, and seaweeds can absorb iron as mineral. Sea desert progresses because this fluvic acid are not supplied from forests and the ocean became lack of iron. 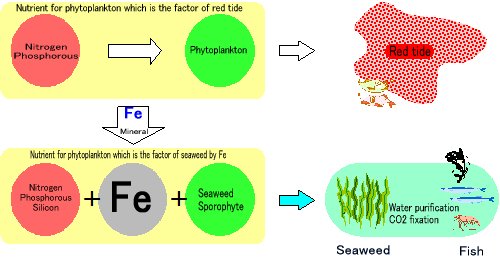 To dissolve iron and make it as ferrous ion, MiraCarbon plays important rule. MiraCarbon are made almost by carbon. Carbon has higher electronegativity compared to irons. Thus, by contacting, iron which is lower electronegativity elutes ferrous ion. By installing MiraCarbon with iron materials in the ocean, ferrous ion is eluted. Seaweeds attached MiraKombu absorbs ferrous ion and grows more. Moreover, ferrous iron chemically reacts with phosphate in the water and produces iron phosphate which blocks the overgrowth of phytoplankton which is the cause of red tide. Also, ferrous iron affects positively on zooplankton and works as important minerals for abalones and oysters.
|
||||||||||||||||||||||
| Copy right©JWG All rights reserved. |